
In a study published in Nature Methods, a research group led by Prof. YE Mingliang from the Dalian Institute of Chemical Physics of the Chinese Academy of Sciences (CAS), collaborating with Prof. LUO Cheng's group from the Shanghai Institute of Materia Medica of CAS, developed a highly sensitive proteomics method called peptide-centric local stability assay (PELSA), which enables the simultaneous identification of ligand-binding proteins and their binding sites in complex systems. PELSA is broadly applicable to diverse ligands including metabolites, drugs, and pollutants.
The biochemical functions of proteins invariably involve interactions with ligands of some type, which act as enzyme substrates or inhibitors, signaling molecules, allosteric modulators, structural anchors, etc. Monitoring protein-ligand interactions is thus essential for characterizing proteins with unknown functions, for investigating regulatory mechanisms in cell metabolism, and for elucidating drug mechanisms of action. Knowledge of the ligand-binding regions is also extremely valuable for structure-based drug design and biological hypothesis generation.
Traditional methods for determining binding sites and affinities typically require the purification of recombinant proteins, which can be both time-consuming and labor-intensive. In addition, purified proteins may not fully replicate their native cellular state, resulting in inaccurate affinity measurements. Modification-based proteomics methods offer a powerful solution for identifying ligand-binding proteins and their sites directly in native cellular lysates. However, they often require ligand modification, which can affect ligand activity and cannot be applicable to ligands that cannot be modified.
In the method proposed in this study, the researchers used a large amount of trypsin (E/S ratio of 1:2) to directly generate small peptides from native proteins. As these peptides are generated under native conditions, their abundance represented a measurement of proteins’ local stability. The large amount of trypsin ensured that even protein segments in low energy states could be cleaved, resulting in the generation of a large number of peptides reflecting protein’s local stability. These peptides were separated from the partially digested proteins, collected and directly analyzed by mass spectrometry. By measuring the peptide abundance in ligand-treated and vehicle-treated samples, the ligand-binding regions and the corresponding binding proteins can then be determined.
PELSA has showed superior sensitivity in target protein identifications. For example, in identifying the target proteins of a pan-kinase inhibitor staurosporine, PELSA showed a 12-fold increase in kinase target identification compared to the state-of-art modification-free method, LiP-MS. Compared to the widely used thermal proteome profiling (TPP) technique, which lacks binding site information, PELSA identified 2.4-fold more kinase targets. Dose-dependent PELSA experiments can measure local affinity, providing insights into the dynamic protein structural changes upon ligand binding under physiological conditions.
Metabolites, known for their structural diversity and often low-affinity binding to proteins, pose challenges. PELSA proved particularly effective for the systematic identification of metabolite-binding proteins. For example, PELSA identified 40 candidate target proteins for alpha-ketoglutarate in HeLa cell lysates, 30 of which were well-known binding proteins of alpha-ketoglutarate, demonstrating the method’s high sensitivity and reliability. In addition, PELSA identified binding proteins for other metabolites, such as folate, leucine, fumarate, and succinate, showcasing its broad applicability.
PELSA can directly detect ligand-induced local stability shifts of proteins in total cell lysate without the need for chemical modification of ligands. It is broadly applicable to diverse ligands, and allows for systematic analysis of ligand-binding proteins, their binding sites, and local binding affinities in cell lysate, where proteins carry physiological post-translational modifications and are associated with interacting proteins.
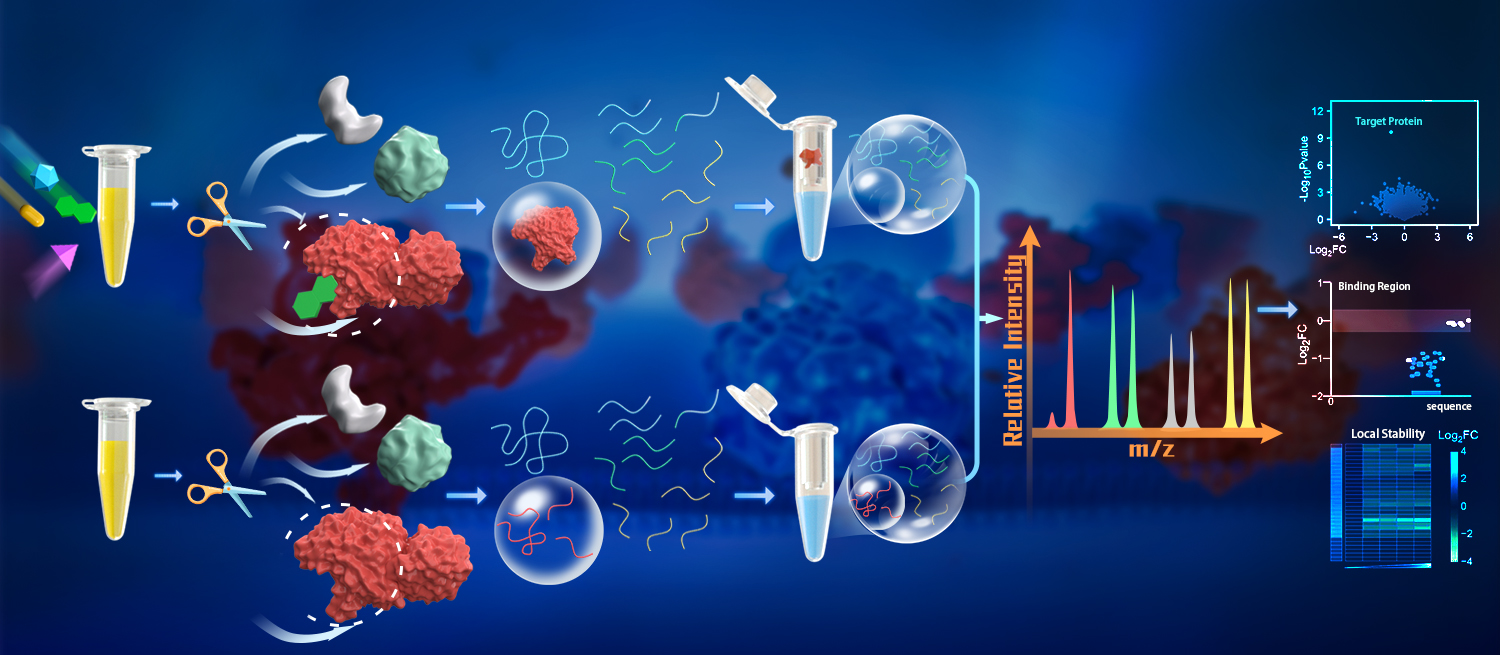
PELSA allows for systematic analysis of ligand-binding proteins, their binding sites, and local binding affinities in cell lysate. (Image by LI Kejia)

86-10-68597521 (day)
86-10-68597289 (night)

86-10-68511095 (day)
86-10-68512458 (night)

cas_en@cas.cn

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

